MHRA UK Responsible Person Mandate
MHRA mandate has made it mandatory to appoint an MHRA UK Responsible Person if the manufacturer of the medical device is not based in the UK and wants to place the device in the Great Britain market. The requirement for MHRA UK responsible person is laid out in the United Kingdom Medical Device Regulations (UK MDR). The MHRA UK Responsible person services include registering the medical devices with MHRA on behalf of the manufacturer, ensuring conformity to be available for inspection, responding to any queries from MHRA, and carrying out Post Marketing Surveillance (PMS) activities.
Role of UKRP
The appointed United Kingdom Authorised Representative should register device products with the MHRA.
Eligibility for being a UKRP
Any third-party entity or an importer or distributor can act as an MHRA UK Responsible Person on a foreign manufacturer’s behalf.
Timeline
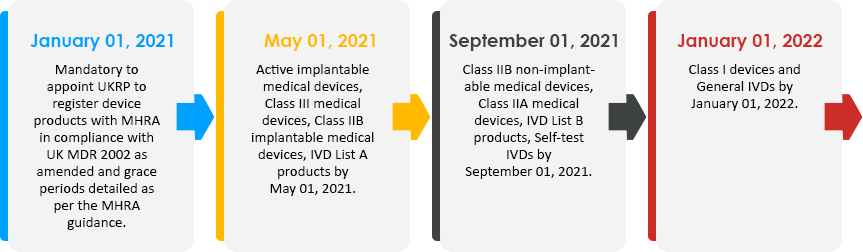
The timeline for appointing a UKRP is in line with the timeline for getting the device registered with MHRA i.e., 4 months, 8 months, and 12 months for different classes of devices.




